SFRI India provides a comprehensive portfolio of Hematology, Biochemistry, and Immunology Instruments, along with their Associated Reagents. Built on performance, quality, and reliability, our product catalog continues to grow and adapt to the needs of laboratories worldwide.
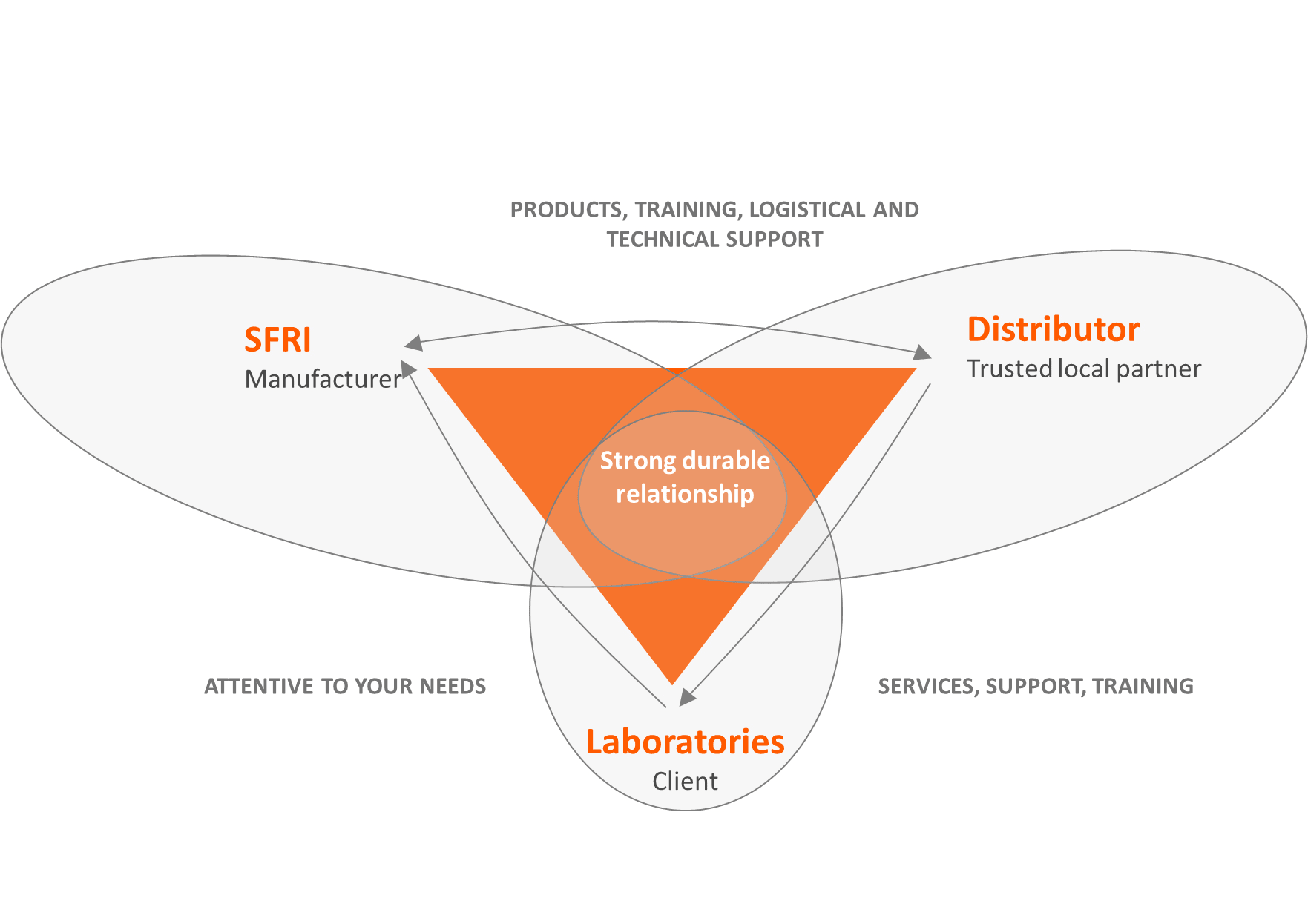
Through consistent feedback from our partners and clients, SFRI India refines its offerings and develops innovative solutions that remain relevant, progressive, and trusted in the ever-evolving field of In-Vitro Diagnostics.
Marketing In-Vitro Diagnostic Medical Devices—such as SFRI Instruments and Reagents—requires CE certification, governed in Europe by Directive 98/79/CE. All SFRI products fully comply with these regulations, and every reagent is registered with the ANSM (Agence Nationale de Sécurité du Médicament, the French equivalent of the FDA).
Quality is more than a priority at SFRI India — it is a daily commitment. Every Instrument Reagent System undergoes extensive testing throughout its lifecycle, from development to market launch.
For SFRI India, quality control is not a formality—it is the guarantee of reliability, performance, and customer trust.
SFRI is committed to sustainable and ethical development. Beyond regulatory requirements, we voluntarily align with global standards, including the UN Global Compact Charter, affirming our dedication to future generations.
As a European company, SFRI complies with REACH, WEEE, and RoHS:
SFRI India translates its commitment to sustainability into action by developing safer, eco-friendly reagents—completely free of cyanide for safer laboratories and a healthier environment.
SFRI actively contributes to the industry as a member of EDMA (European Diagnostic Manufacturers Association) and SIDIV (In-Vitro Diagnostic Industry Union).